Introduction
The liver plays a critical role in maintaining homeostasis through various metabolic, synthetic and detoxifying processes making it one of the primary organs studied for human disease modeling and drug discovery experimentation. At the cellular level, hepatocytes constitute approximately 80% of the liver mass while non-parenchymal cell (NPC) types make up an additional ~ 6% of the overall organ.1
Hepatocytes are a key cell type for toxicity and drug metabolism testing, increasingly performed with liver-on-a-chip approaches. Due to the sensitive nature of these types of experiments, accurate levels of hepatocyte seeding are required to obtain reproducible results. Both manual and traditional cell counters experience challenges counting hepatocytes due to irregular size and shape, multiple nuclei, autofluorescence, frequent clumping and cell debris. To address the needs of these researchers, DeNovix developed advanced machine learning techniques to create unique algorithms capable of reliably reporting concentration and viability of hepatocyte samples. In addition, the application provides quality information including the number of non-hepatic cells or non-cellular debris present in the specimen (Figure 1).
This technical note demonstrates a highly reliable cell counting and viability assessment of Hepatocyte, Kupffer, Stellate, and Liver Endothelial cells using the DeNovix CellDrop™ FLi Automated Cell Counter.
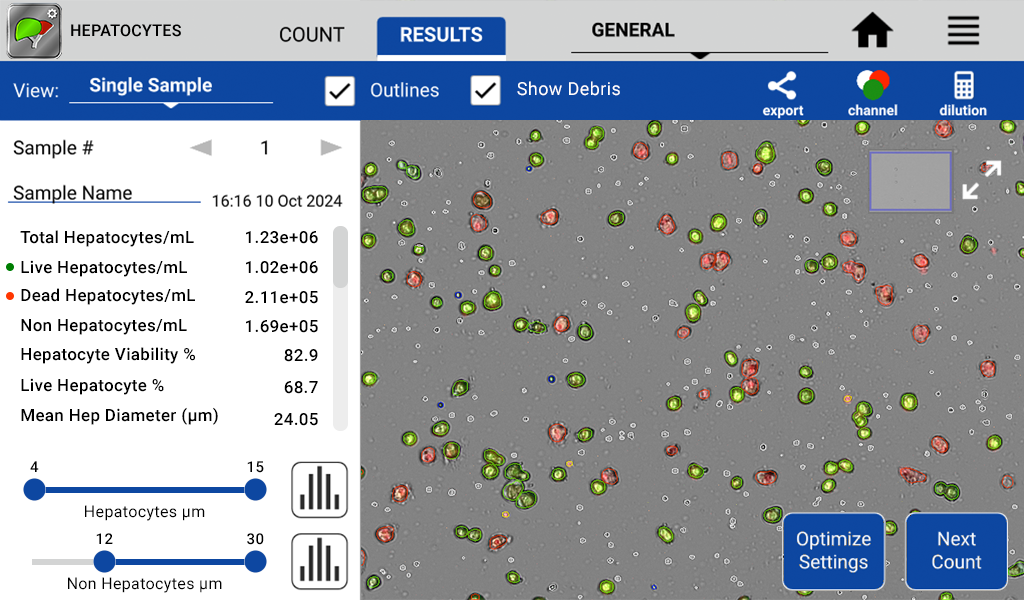
Figure 1. Image of the CellDrop accurately identifying and enumerating live hepatocytes (green), dead hepatocytes (red), free nuclei (yellow outline), lymphocytes (blue outline) and erythrocytes or other debris (white).
Methods
Hepatocytes
To demonstrate the accuracy and reproducibility of the CellDrop Hepatocyte App, three species of isolated hepatocytes (canine, human and rat) were selected (BioIVT, Baltimore MD). These were thawed in accordance with the manufacturer’s instructions and resuspended in 2 mL of Invitrogro KHB buffer (BioIVT, Baltimore MD).
From this initial resuspension, 2 serial dilutions were made by adding 1 mL of this resuspension to 1 mL of KHB buffer for a total of 3 samples. The samples were then stained with equal volumes of stain:cells using either AO/PI (DeNovix, Wilmington DE) and counted on a CellDrop equipped with the Hepatocyte app or stained with Trypan Blue (DeNovix, Wilmington DE) and counted on a manual hemocytometer using a brightfield microscope. Each sample was counted with 3 replicates.
Note: Best results are obtained by using 50 uL of AO/PI to 50 uL of hepatocytes and agitated gently by rocking the tube.
Non-Parenchymal Cells
Cryopreserved Primary Human Liver Endothelial cells (LECs), Stellate cells (SCs) and Kupffer cells (KCs) were obtained (LifeNet Health LifeSciences, Virginia Beach VA) and stored in vapor phase liquid nitrogen until ready for use. At the time of experiment, primary human Stellate, and Kupffer cells were thawed and processed.2 Primary human liver endothelial cells were similarly thawed but maintained in T-75 flasks until approximately 85% confluent. NPCs were then resuspended in NPC* medium, stained with AO/PI (DeNovix, Wilmington DE) and loaded onto the CellDrop FLi to determine “%Viability” and “Live Cells/mL” counts in 3 replicates. Specifically, SCs were counted on the Hepatocyte app, whereas KCs and LECs were counted on the Primary Cell AOPI app.
* Denotes a modified recipe without ITS + premix (#354352, Corning)
Results
Automated Counting of Hepatocytes
The CellDrop Hepatocyte App and the manual hemocytometer counts showed a very close relationship with all but one of the dilutions, showing non-statistically significant differences in the counts of live hepatocytes/mL (p value < 0.02). The one dilution set that did show a significant difference was the dilution that had the lowest overall counts, which may account for this result. The degree of overlap between the two methods over the range of concentrations is high, and in both CellDrop and manual counts, the r2 values are all greater than 0.99. Also note that the standard deviation on the CellDrop is lower than the manual hemocytometer (Figure 2a). Likewise, the viability numbers between the two methods appears to be very close with no statistically significant differences between any of the nine replicates as shown in Figure 2b (p value < 0.02).
View this data and more in Technical Note 228: Automated Counting of Hepatocytes.
Figure 2. a) Comparison of the concentration of live cell/mL for human hepatocytes diluted and counted on both a CellDrop Automated Cell Counter and a manual hemocytometer. *Denotes statistically significant difference. b) Comparison of the percent viability of each dilution of human hepatocytes.
Automated Counting of Liver NPCs
NPCs were successfully counted on the CellDrop FLi with viabilities in these experiments ranging from 59.4% to 94.8% and live cell concentrations of 4.42 x 106 – 5.33 x 106 cells/mL. The Hepatocyte app, which deploys machine learning algorithms trained on hepatocyte datasets, optimally counted live Stellate cells (Figure 3). The Primary Cell AO/PI App was used to count Kupffer cells (Figure 4) and Liver Endothelial cells (Figure 5).
Figure 3. Percent viability and live cell/mL counts of primary human stellate cells. On left; cells stained with AO/PI along with efficient identification of live (green) and dead (red) cells in the Hepatocyte app. On right; % viability (dark red) and live cell/mL concentrations (light red) from 3 replicates.
Figure 4. Percent viability and live cell/mL counts of primary human Kupffer cells. On left; cells stained with AO/PI depicting live (green) and dead (red) cells. On right; % viability (dark green) and live cell/mL concentrations (light green) from 3 replicates.
Figure 5. Percent viability and live cell/mL counts of primary human liver endothelial cells. On left; cells stained with AO/PI indicating live (green) and dead (red) cells. On right; % viability (dark gray) and live cell/mL concentrations (light gray) from 3 replicates.
Conclusion
When pursuing reliable and reproducible liver-on-a-chip models, accurate cell counting is of paramount importance. The data presented demonstrate that the DeNovix CellDrop Automated Cell Counter provides a critical improvement in this area. CellDrop Hepatocyte counting is trained and validated across multiple species, enabling researchers to rapidly and reliably count cells, assess viability and obtain additional quality metrics related to the sample. Moreover, its ability to accurately enumerate non-parenchymal cells, including Kupffer, Stellate, and Liver Endothelial cells assures a comprehensive analysis of liver-on-a-chip components.
Live Demo
Want to see how it works for yourself? Get a quick look at the live preview and hepatocyte count data available on the Hepatocytes app.
References
- Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol. 2013 Apr;3(2):567-98. doi: 10.1002/cphy.c120011.
- Emulate Protocol: “Liver-Chip Quad-Culture Protocol, EP-226 Rev. B”
Count Cells Without Slides
If you’re interested in learning more about the CellDrop Automated Cell Counter, consider signing up for a 7-Day Free Trial, scheduling a call with our applications team, or requesting a quote. Click the buttons below to take the next step!
05-DEC-2025
INVITROGRO™ is a registered trademark of BioIVT and is used for identification and references purposes only. DeNovix, DeNovix products, and this website are not endorsed or authorized by, or in any way affiliated with BioIVT.