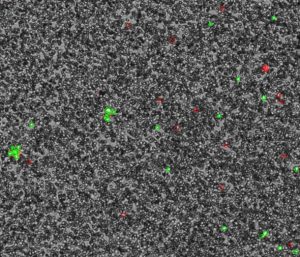
Preparing Whole Blood for Counting on CellDrop
Recommended Whole Blood Protocol
CellDrop count algorithms use multiple parameters, together called a count protocol, to allow a user to fine tune what is counted as a cell. Although some adjustments may still be required, the recommended settings for counting PBMCs in whole blood are presented in Table 1.
Table 1: Recommended Protocol Settings for Whole Blood| Count Application | AO/PI |
| Chamber Height | 100 µm |
| Dilution Factor | 200* |
| Diameter(min) | 4 µm |
| Diameter (max) | 15 µm |
| Live Roundness | 1 |
| Dead Roundness | 1 |
| Green Fluorescence Threshold | 1 |
| Red Fluorescence Threshold | 1 |
* Adjust to reflect the actual dilution as required
Ensuring Reproducible Results
The following tips will help ensure that the data from whole blood counts on the CellDrop is consistent and accurate:
10-APR-2023