Introduction
In brewing, high-viability yeast plays a critical role in converting sugars into alcohol, driving both the pace and purity of the process. When yeast viability declines, fermentation processes can stall or produce off-flavors, disrupting product consistency.
Monitoring yeast viability isn’t just good practice—it’s central to maintaining control over the chemistry of beer and critical for consistent fermentation performance and high-quality beer production. Traditional methods for measuring yeast viability, such as using light microscopy with a hemocytometer and colorimetric stains, can be time-consuming, inaccurate, and inconsistent across users.
Using traditional viability stains like Methylene Blue to distinguish between recently dead cells and live cells accurately can make it challenging to assess yeast cell viability. This is due to some living cells with damaged membranes incorrectly staining, leading to inaccurate viability estimations. 1,2
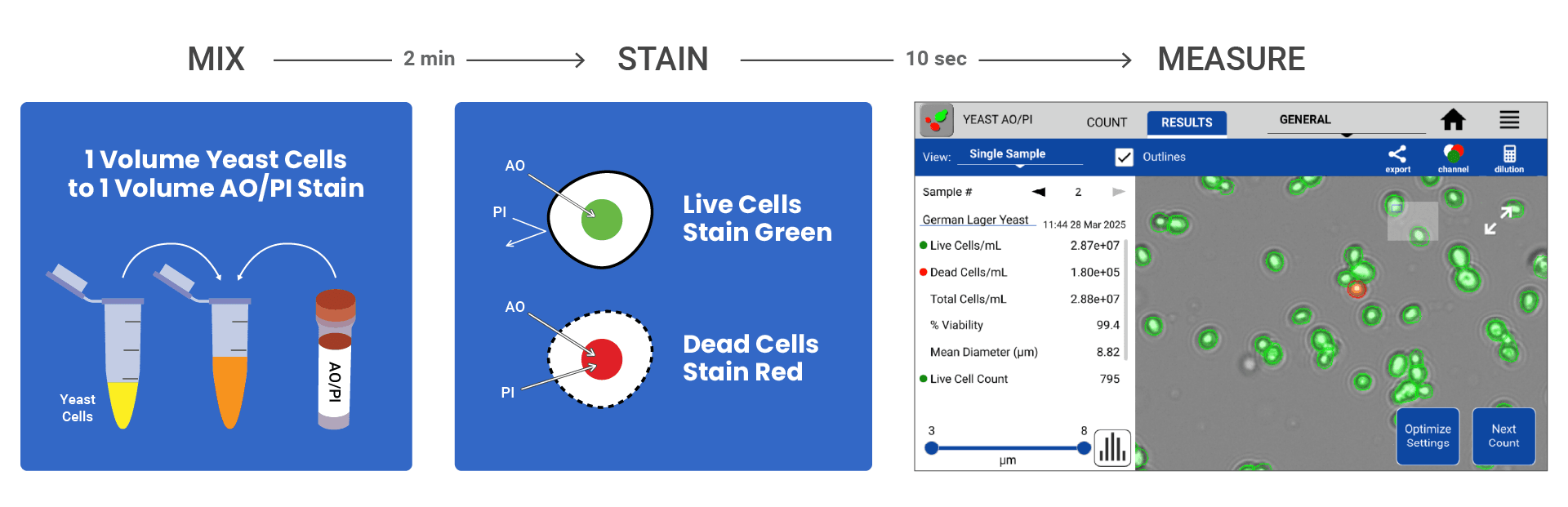
In Figure 1, a novel approach to yeast viability is introduced, leveraging the power of fluorescent dyes in the DeNovix Acridine Orange / Propidium Iodide (AO/PI) Viability Assay in conjunction with the CellDrop FLxi Automated Cell Counter. This innovative method offers breweries a cost-effective rapid fluorescent detection method and provides a clear distinction between live and dead yeast cells, allowing users to count viable cells, dead cells, and total cell numbers accurately.
Methods for Accurately Counting Live and Dead Yeast Cells
Briefly, German Lager Yeast cell samples were acquired from White Labs, San Diego, USA (Figure 2., Part no: WLP830). An overnight inoculation was made with 2.5 mL of fresh YPD and 66.5 uL of the German Lager Yeast stock on a shaker at 300 rpm and 30 degrees Celsius. After overnight inoculation, yeast samples were diluted using PBS buffer or deionized water to a concentration between 1.70E+04 to 1.00E+08. The AO/PI dye was added to yeast cells in a 1:1 ratio (50 uL of diluted yeast sample and 50 uL of DeNovix AO/PI) before counting using the CellDrop FLxi Yeast AO/PI App.
Results
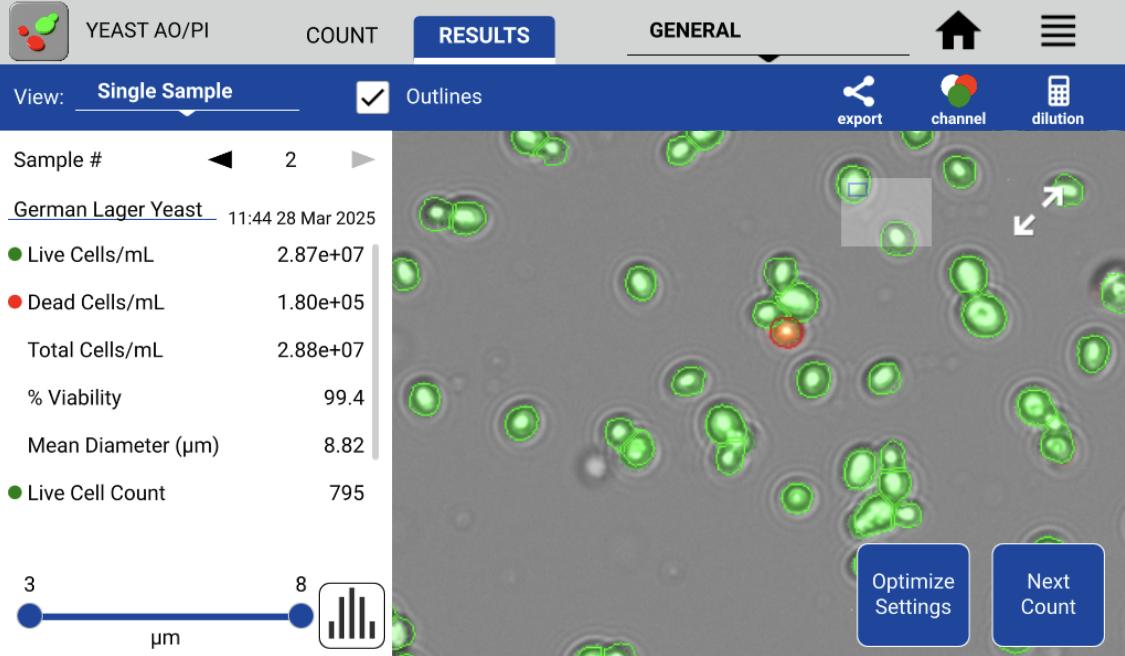
Accurate and efficient yeast counting and viability assessment are paramount for successful brewing. Figure 2 shows the Results tab of the CellDrop software, which displays after each cell count. Live cells/mL, Total cells /mL, and percent viability of each sample are automatically calculated.
The AO/PI Viability Assay clearly distinguishes between live cells (green) and dead cells (red). This minimizes subjectivity and improves accuracy, providing a significant advantage over the traditional colorimetric dyes. Moreover, the cells/mL calculations for both live and dead cell counts are color-coded with their respective colors for the fast referencing of viability information. All data is also automatically saved on the instrument and available for recall at a later date if necessary.
Results
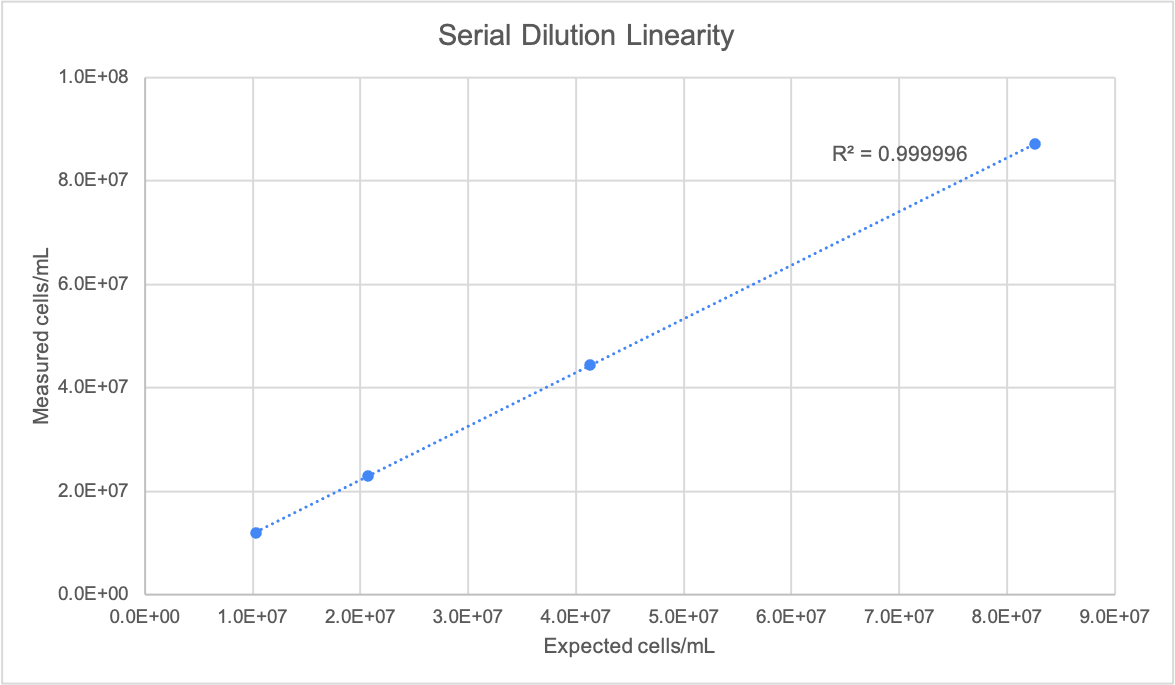
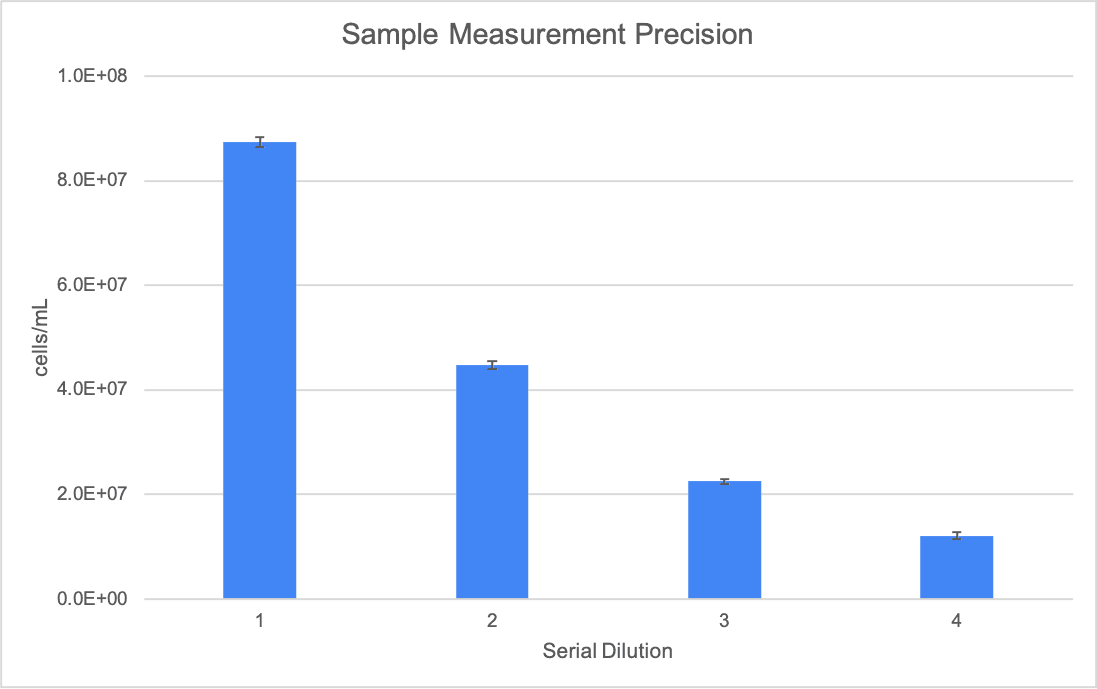
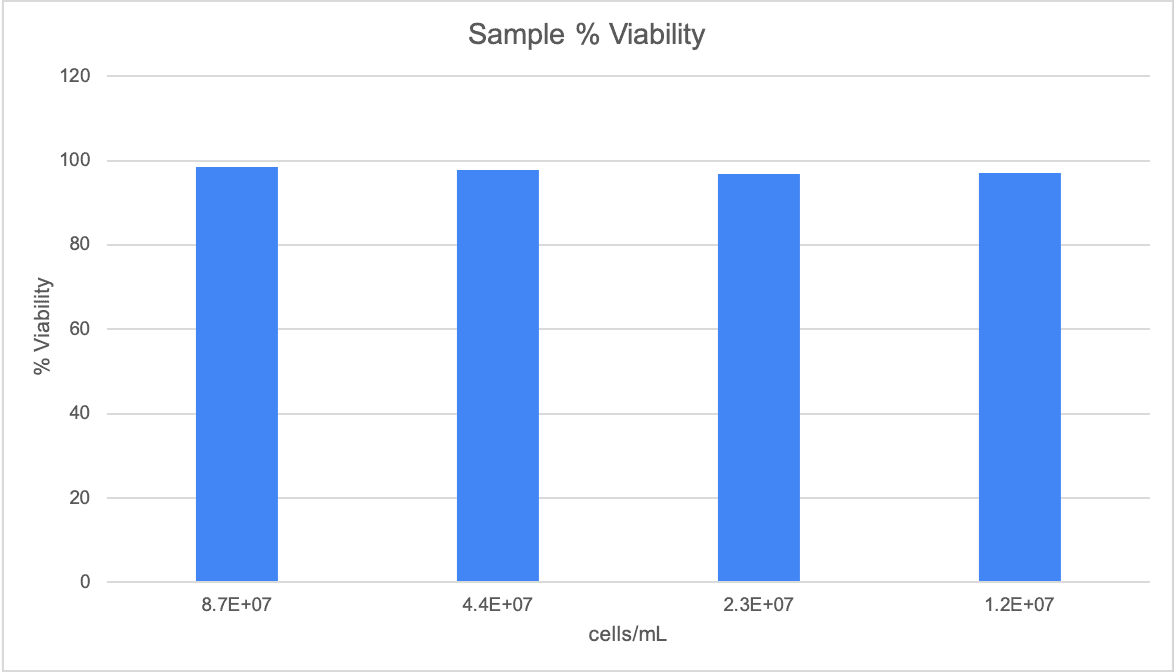
A stock solution of German Lager yeast sample was prepared with a target concentration of 8.26E+06 cells/mL. Three additional dilutions were prepared from this stock with target concentrations of 4.13E+06 cells/mL, 2.07E+06 cells/mL, and 1.03E+06 cells/mL. Using the Yeast AO/PI App, total cell counts and % viability were measured, with five replicates of each concentration counted. Figures 3, 4 and 5 detail the linearity, precision, and viability consistency across serial dilutions. Following the same protocol, similar performance was achieved using other brewing yeast strains such as French Saison.
Conclusion
This method is particularly helpful for brewers monitoring changes in yeast cell concentration throughout fermentation processes.
The CellDrop FLxi, with its user-friendly interface and optimized Yeast AO/PI app, streamlines the yeast counting process. The device provides a rapid and reliable solution for breweries and wineries seeking to optimize their yeast management practices. The data presented here, using German Lager yeast as a model, highlight the potential of this approach to enhance fermentation control and contribute to the production of high-quality beverages. The method demonstrated is applicable to a wide range of yeast species and other cell types. The DeNovix AO/PI Assay (cat #CD-AO-PI-1.5) and the Yeast AO/PI app on CellDrop FLxi enable rapid automated cell counting for yeast samples.
Click here to learn more about the CellDrop Automated Cell Counter or request a quote.
Resources
- Luarasi, L. (2016). The Relationship Between Yeast Viability and Concentration in the Fermentation Process of Wort Beer Production. https://www.idpublications.org/wp-content/uploads/2016/03/Full-Paper-THE-RELATIONSHIP-BETWEEN-YEAST-VIABILITY-AND-CONCENTRATION-IN-THE-FERMENTATION.pdf
- White, C., Zainasheff, J. (2010). Yeast, The Practical Guide to Beer Fermentation. Brewers Publications