Introduction
Plant protoplasts are used in a variety of ways in plant science and are an experimental system for cell biologists to explore the structure, chemistry, and function of plant cells. Entire plant regeneration is possible with protoplasts, as they have the ability to divide after creating a single cell wall. Plant tissue is then developed, thus allowing regeneration. The focus of this technical note is to give a detailed description of how to optimize the AO/PI or Custom Methods Applications for counting protoplasts on the DeNovix CellDrop™ FL or CellDrop FLi Automated Cell Counters in conjunction with fluorescein diacetate (FDA) and propidium iodide (PI) fluorescent dyes.
Traditional brightfield counting of protoplasts is time-consuming as well as challenging due to the visible cell structure in brightfield. Therefore, dual-color fluorescence is recommended for counting and viability determination. The AO/PI App and the Custom Methods App are both appropriate for counting nucleated live cells fluorescing green (FDA) or dead cells fluorescing red (PI). For the purposes of this tech note, the AO/PI app was used.
Materials and Methods
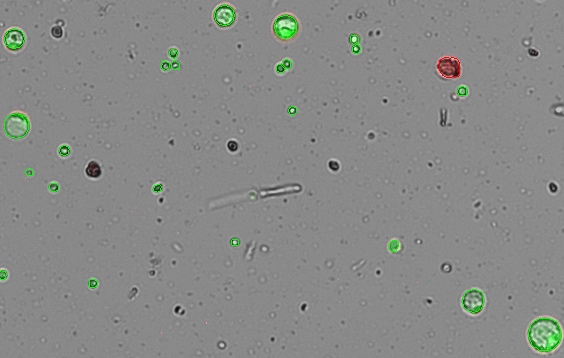
This staining protocol is adapted from a method published by Lin et al. in Frontiers in Plant Science (1). FDA (Sigma-Aldrich cat #F7378) was dissolved in acetone to make 5 mg/mL stock solution. PI (Sigma-Aldrich cat #P4864) was diluted to 0.5 mg/mL in 0.4 M D-mannitol. The working solution was made fresh by adding 10 µL FDA and 10 µL PI in 0.5 mL of 0.4 M D-mannitol. The solution has a finite lifetime of about 30 minutes. Fresh solution should be prepared after 30 minutes. 5 µL of working solution was added to 10 µL of cell solution. The cells were allowed to incubate for 3-4 minutes. The cell solution was gently mixed, and a 10 µL aliquot was dispensed into the CellDrop counting chamber, and the cell sample was counted in the green and red fluorescent channels (Figure 1). A cell counting protocol was prepared based on the dilution factor applied and the expected diameter of the protoplasts.
Refer to Tech Note 186 – CellDrop Best Practices for additional guidance on sample loading, proper focus, and proper exposure. See www.denovix.com/celldrop-videos for more information.
Protoplast Protocol Settings
Table 1: Count Settings
| Count Application | AO/PI or Custom |
| Chamber Height | 100 µm |
| Dilution Factor | 1.5 |
| Diameter (min) | 6 µm |
| Diameter (max) | 55 µm |
| Live Roundness | 1 |
| Dead Roundness | 1 |
| Green Fluorescence Threshold | 1 |
| Red Fluorescence Threshold | 1 |
Results and Summary
The CellDrop reports data for total, live, and dead cell counts in terms of counted cells and cells/mL, as well as mean cell diameter and percent viability when applicable. The CellDrop FL with dual color fluorescence has powerful counting algorithms and customizable count settings to enable accurate counting and viability determination of a wide variety of cell types, including plant protoplasts.
1. Lin HY, Chen JC, Fang SC. A Protoplast Transient Expression System to Enable Molecular, Cellular, and Functional Studies in Phalaenopsis orchids. Front Plant Sci. 2018 Jun 22;9:843. doi: 10.3389/fpls.2018.00843. PMID: 29988409; PMCID: PMC6024019.
21-MAR-2025